One of the critical lethal features of COVID was cytokine storm, a severe imbalance of pro-inflammatory chemical mediators. Similar imbalances are seen in sepsis, and a a more chronic, debilitating version as autoimmune diseases. Our understanding of inflammatory homeostasis, which balances pro and anti-inflammatory forces, has implicated more than just our immune cells. A new study in Nature demonstrates the connection between body and brain mediated through the vagus nerve.
The vagus nerve derives its name from the Latin vagary, meaning wandering. It does just that, connecting with motor and sensory fibers in our throat, esophagus, intestines, both large and small, heart, and lungs. Not only is it the largest nerve in our autonomic nervous system, but it is also the largest of the cranial nerves, those that directly connect to the brain.
“We identified vagal neurons that respond to pro-inflammatory versus anti-inflammatory immune mediators, and showed that they signal to a genetically defined population of neurons in the brainstem to modulate and shape the course of an inflammatory response. These results reveal the influence of the body-brain axis in controlling innate immunity…”
The researchers made those connections and findings in a series of studies in mice using various sophisticated techniques to image and isolate each phase in the process. Let’s try to summarize.
Using a portion of the outer membrane of Gram-negative bacteria, the lipopolysaccharide (LPS), a standard stimulus promoting immune activation the research demonstrated increasing levels of both pro and anti-inflammatory chemical mediators, 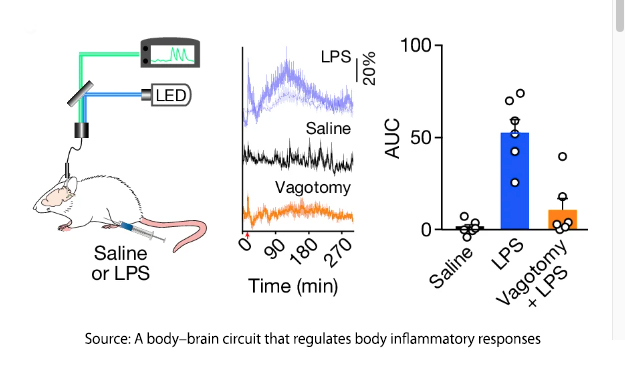 cytokines. They scanned the mice's brains and identified a rising degree of neural activity in a specific area of the brainstem, the caudal nucleus of the solitary tract. (cNST) – the first stop in the brain for information from the vagus nerve. There was no similar increase in activity when the mice were injected with saline; more importantly, cutting the vagus nerve, a vagotomy, prevented activation of the cNST. There is a vagal-cNST immune axis.
cytokines. They scanned the mice's brains and identified a rising degree of neural activity in a specific area of the brainstem, the caudal nucleus of the solitary tract. (cNST) – the first stop in the brain for information from the vagus nerve. There was no similar increase in activity when the mice were injected with saline; more importantly, cutting the vagus nerve, a vagotomy, prevented activation of the cNST. There is a vagal-cNST immune axis.
Using a bit of fancy genetic alterations, [1] the researchers could control the activation or inhibition of those cNST neurons. Inhibition of the neurons led to “a runaway, out-of-control inflammatory response.” Pro-inflammatory cytokines rose 3-fold, while anti-inflammatory cytokines declined 3-fold. Excitation of the neurons provoked the opposite response, with anti-inflammatory mediators rising tenfold and pro-inflammatory mediators reduced by 70%. However, these effects only occur in the presence of an ongoing immune response; if the immune system is not being challenged, the neurons remain “silent.” So those cNST neurons are an off-on dial to our immune response
The researchers then turned their attention to the nodose ganglion, a portion of the vagus nerve that collects sensory information from the heart, lungs, and intestines, among other sites. The nodose ganglion is known to be involved in the homeostatic mechanisms of digestion, heart rate, blood pressure, and breathing. Here, they found that “anti-inflammatory and pro-inflammatory cytokines activate two discrete non-overlapping populations of vagal sensory neurons.”
“cytokines themselves function as an immune mediator in the body–brain axis, with the vagal neurons functioning as the conduit transmitting the inflammatory information to the cNST.”
In response to anti-inflammatory cytokines, one group of nodose ganglion neurons acts on those cNST neurons to enhance the anti-inflammatory response and dampen the pro-inflammatory state. The other group of neurons signals to the cNST more strategically down-regulate pro-inflammatory markers, fine-tuning our immune response.
In their final experiments, the researchers looked at the impact of vagal activity on two diseases. Using those previously mentioned lipopolysaccharides, they administered a lethal dose to a group of unfortunate mice. Chemogenetically activating those vagal neurons or the cNST neurons themselves “dramatically transform[ed] the survival of these animals to an otherwise lethal dose of LPS: approximately 90% of the mice were alive after the immune challenge.” In the second experiment using a mouse model of ulcerative colitis, activation of those vagal anti-inflammatory neurons protected the animals from “dramatic damage to the distal colon, … significant occult stool blood and … a major increase in the levels of pro-inflammatory cytokines.”
The researchers conclude:
“We propose that the cNST neurons function as a biological rheostat controlling the extent of the peripheral inflammatory response by exerting positive-feedback and negative-feedback modulation on immune cells.”
The discovery of the vagus nerve's role in modulating inflammation through the caudal nucleus of the solitary tract (cNST) neurons opens new avenues for treating inflammatory diseases. By tweaking this body-brain axis, scientists can potentially control runaway inflammatory responses, offering hope for conditions from sepsis to ulcerative colitis. It's a reminder that sometimes, the solutions to our most pressing health issues are right under our noses — or rather, deep inside our heads.
[1] Mouse strains were genetically altered to express a tamoxifen-dependent recombinase CreER in an activity-dependent manner from various genes. Active cells that express CreER undergo recombination when tamoxifen is present, whereas inactive cells do not. These genetically encoded effectors allow visualization and manipulation of neurons, facilitating “dissection of neural circuit function” while maintaining a living creature.
Source: A body–brain circuit that regulates body inflammatory responses Nature DOI: 10.1038/s41586-024-07469-y



