There are cancer scares and cancer scares. Many, if not most, result from an unsavory gmish of garbage science—flimsy epidemiological studies (talc) or conflation of hazard versus risk (glyphosate) and what I'll call the "sleazebag legal extortion complex."
It's easy! Lawyers hold up companies that make a product with even a remote or theoretical chance of being carcinogenic. Companies, looking to avoid multi-billion judgments, fold up like wet cardboard. The facts don't matter; a product that carries even a rumor of carcinogenicity is fair game. Ethically challenged environmental groups such as NRDC and the Environmental Working Group cheerfully participate in the "all chemicals cause cancer" mantra (1).
But there is a particularly interesting lawsuit in the news that may have merit. Hector Corvera, a hairstylist, who after 40 years, developed bladder cancer (resulting in the removal of his bladder) may have a legitimate claim. Corvera blames a chemical called paraphenylenediamine – a component of hair dye. While it is impossible for Corvera to prove that the chemical caused his cancer the science, especially chemistry, gives him some pretty firm ground to stand on.
The "meteoric surge in cancer" is false
It is convenient, perhaps even fashionable, to argue that we are now now swimming in a sea of toxins and are thus coming down with cancer like never before. But the data say otherwise:
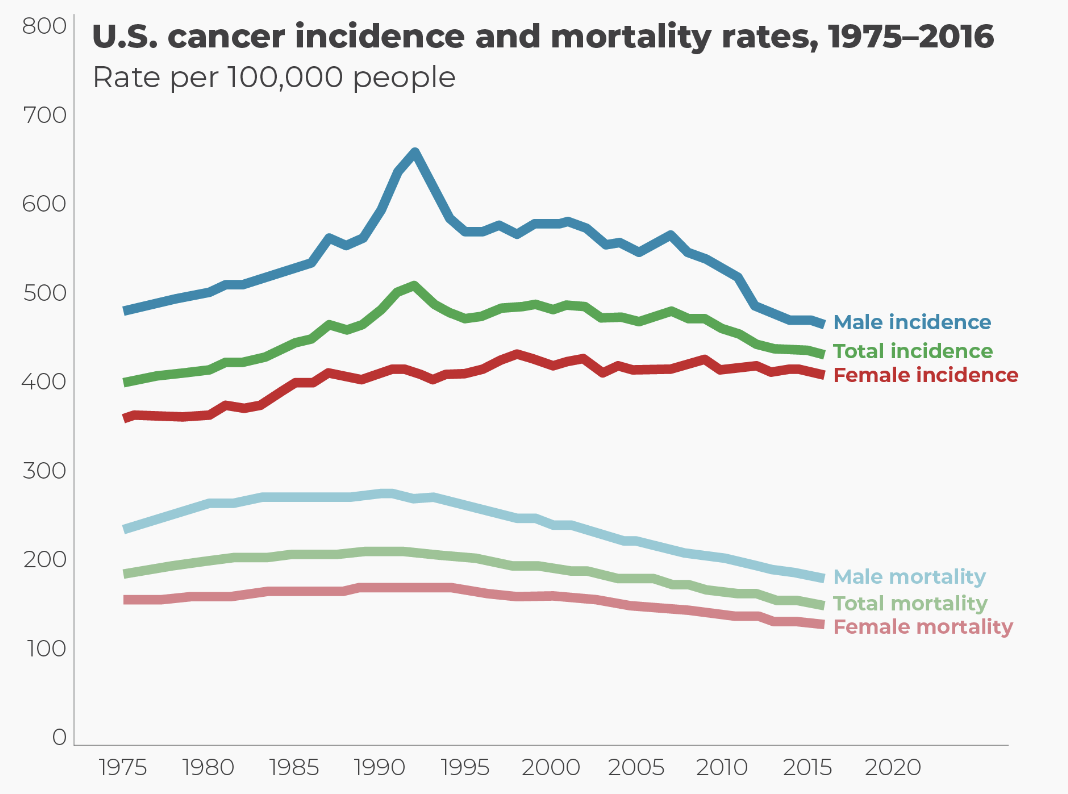
The incidence (2) of cancer in the US has been steadily falling since 1990. Image: HumanProgress
Hey, look! ---> Quiz: Can anyone explain that spike in men's cancer in the early 90s? (Put your answers in the comments section).
This does not mean that all chemicals used by consumers are safe. Some are downright deadly, especially for occupational workers who are constantly exposed to a possibly carcinogenic chemical that would be very unlikely to affect the rest of us.
Irony time
It's almost difficult to believe that neither paraphenylenediamine nor acetaminophen – a drug I've written about for years – is toxic or carcinogenic. But both chemicals are stable and probably harmless. That is until the two chemicals are oxidized. Then, all bets are off.
The irony involves not only the requisite oxidation but also that the two chemicals form a remarkably similar structure that is certainly known to be toxic (from acetaminophen) and a possible carcinogen (from paraphenylenediamine).
How can this be?
Pardon the chemistry, but it's necessary here. I'll try to keep it as inoffensive as possible.
There is a sub-class of organic chemicals called electrophiles (Figure 1). The name is fitting. They love electrons. Electrophiles have an atom (usually carbon) with a partial positive charge. Since electrons are negative it is not difficult to see the attraction. There are tens of thousands of reactions of this type used in organic chemistry. This is perfectly fine in a chem lab, but in your body - not so much.
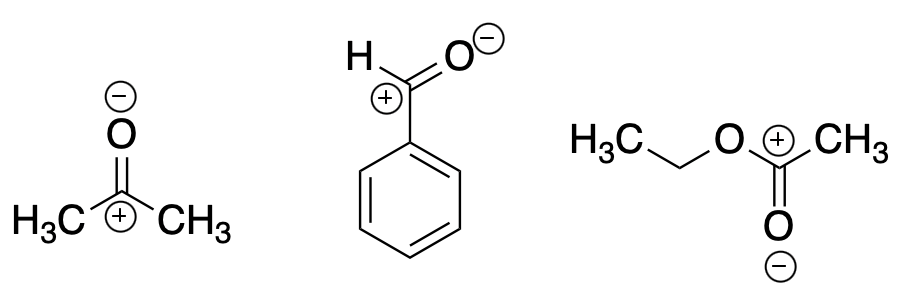
Figure 1. Some common electrophiles (left to right): Acetone, benzaldehyde, and ethyl acetate. Note the partial positive charges on carbon and partial negative charges on oxygen. Can anyone explain why this occurs?
What does this have to do with Tylenol or hair dye?
Plenty. Figure 2 shows the oxidation of both acetaminophen and p-phenylenediamine. Note that the oxidation products are very similar in structure; the products are very potent electrophiles, which means they form similarly highly reactive (3) transient intermediates. In the case of acetaminophen, the toxic metabolite is N-acetyl-p-benzoquinone imine, which has mercifully been given the nickname NAPQI. In the case of paraphenylenediamine, the toxin formed is p-benzoquinone diimine. To an experienced organic chemist, both of these look like trouble.
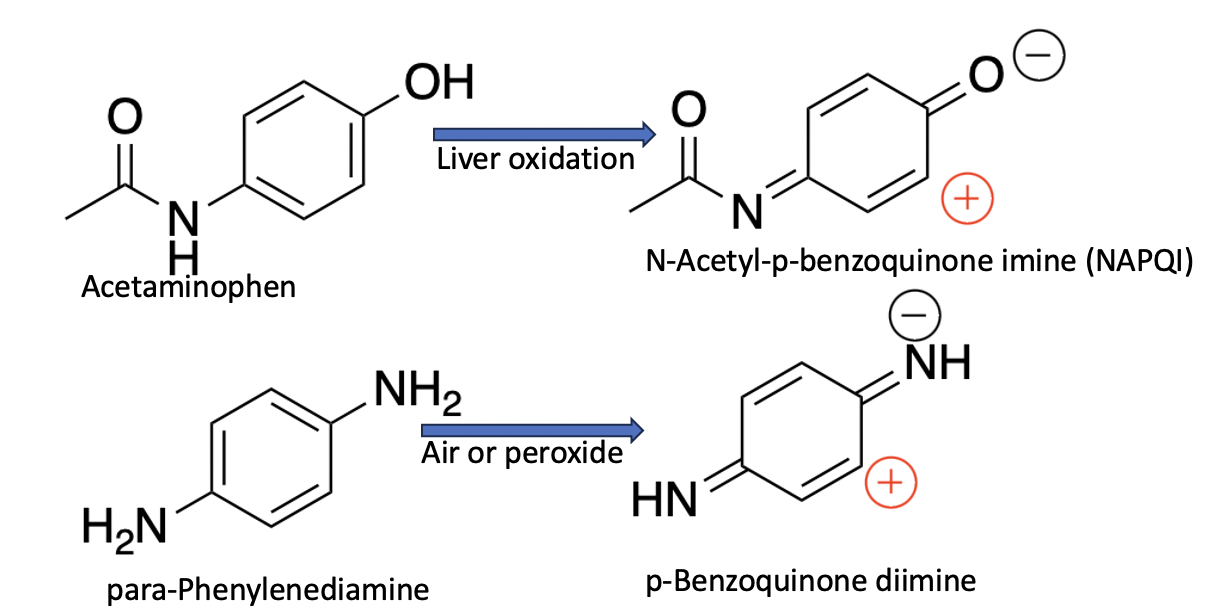
Figure 2. The oxidation of both acetaminophen and p-phenylenediamine leads to short-lived, highly reactive toxins. The most reactive part of each molecule is indicated by a red plus sign. The similarities in structure and function should be clear.
Why is this important? DNA damage
As I mentioned earlier, electrophiles react with nucleophiles - molecules with electrons to donate, often nitrogen and sulfur, which means proteins and DNA. This can be a serious problem if they do so within your body. The electrophiles are impatient; they want electrons and fast. Where they get them is the problem. Figure 3 should make this obvious.
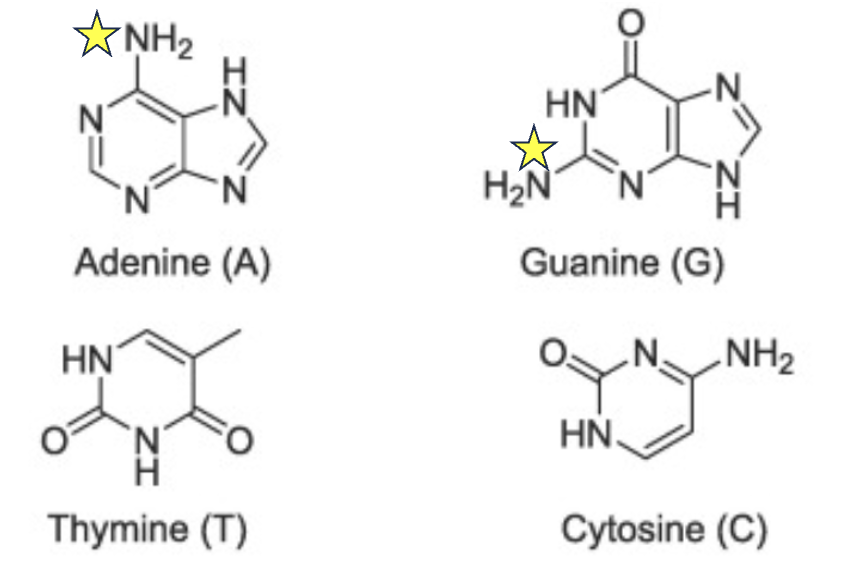
Figure 3. DNA nucleobases, especially adenine and guanine have nucleophilic nitrogen atoms (yellow stars) and are therefore susceptible to attack by electrophiles. The stars indicate the nitrogen atoms in DNA most likely to react with electrophiles like NAPQI.
Potent electrophiles modify DNA by adding unwanted groups to its nucleobases, mainly guanine (G) and adenine (A). This can cause mutations, strand breaks, and DNA crosslinks. DNA mutations are the hallmark of cancer.
Is the association of para-phenylenediamine and cancer real?
It is difficult to prove that a given chemical causes cancer in humans. An article by the American Cancer Society sums this up with regard to hair dyes:
Most studies of people exposed to hair dyes at work, such as hairdressers and barbers, have found a small but fairly consistent increased risk of bladder cancer. However, studies looking at people who have their hair dyed have not found a consistent increase in bladder cancer risk.
In other words, as is so often the case, it is occupational exposure to a given chemical that causes problems, possibly cancer while occasional exposure does not. To paraphrase Paracelus, "the dose makes the carcinogen."
Keep in mind that para-phenylenediamine, unlike acetaminophen, is metabolized before it gets to the liver. The culprits are air and (better) hydrogen peroxide, which is used in the dyeing process. So, there is little question that Mr. Corvera was exposed to both paraphenylenediamine and its oxidative form
Bottom line
What seems to be an enormous coincidence is anything but once you understand the chemistry and metabolism involved. Both chemicals share a similarity in structure and both undergo a similar transformation.
This is nothing new. There are plenty of drugs and chemicals that are metabolically activated, sometimes a harmful process and sometimes helpful (e.g. pro-drugs). This is why pharmacokinetics – the action of the body on the drug – is so critical in evaluating the safety (and for drugs, efficacy) of chemicals.
Did Hector Corvera's bladder cancer come from one of the chemicals in hair dye? We'll never know, but in my opinion, the answer lies somewhere between plausible and likely. If I'm on a jury (4) in the roundup trial Bayer doesn't pay out a cent. For this trial, that could be quite different.
NOTES:
(1) One of many examples of EWG lies in the characterization of BPA, a component of some plastics, which has been used since the 1940s. An extensive, four-year study by the FDA found no evidence of any harm. Yet, the EWG won't rest until it finds something wrong with the stuff.
(2) The graph illustrates cancer incidence, measured as the number of new cases. Incidence is a better measure than the number of cancer deaths because it reflects the actual rate of new cancer cases, providing insight into risk factors, early detection, and the effectiveness of prevention strategies, whereas cancer deaths are influenced by treatment success and survival rates.
(3) Reactive molecules are generally bad news because they indiscriminately react with proteins and genetic material. Many cytotoxic cancer drugs are reactive molecules, which explains some of the nasty side effects.
(4) I won't be on a jury for something like this. Ever. In fact, I managed to get kicked off of one during voi dire. It was a drug case and seeing my background the prosecutor asked whether, as a chemist, I would be able to accept testimony from an expert witness. My answer was "only if he's right." I think I was home in 30 minutes.



