There can't be anyone who hasn't seen horrific footage of the LA fires. Nor could anyone miss seeing plane after plane of some mysterious red liquid dumped on unburnt trees. What is it? Why is it red? How does it work?
Some simple chemistry can answer all these questions.
The red color
The most noticeable feature of Phos-Chek LC95 is its bright red color, but this has little to do with fire suppression. Instead, the color ensures that pilots can easily see where the retardant has been applied, allowing for continuous and accurate coverage.
The red hue primarily comes from iron oxide, a naturally occurring mineral also known as rust, which ranges in color from red to reddish-brown depending on particle size. In some formulations, a food-grade red dye is added to enhance visibility, particularly in challenging terrains or low-light conditions. Both iron oxide and food dyes used in Phos-Chek are non-toxic and environmentally safe (1).
The chemistry of fire suppression
This is where some clever chemistry comes in. Aside from water, the only chemical of consequence is ammonium phosphate, a common fertilizer with low toxicity. Here's how it works.
When ammonium phosphate is heated it decomposes into phosphoric acid and ammonia gas:
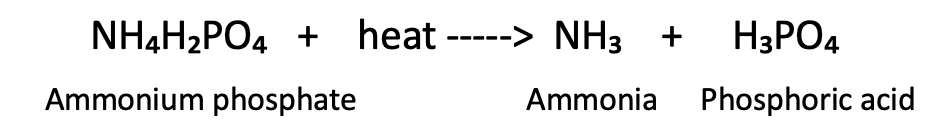
With more heat phosphoric acid further breaks down in a two-step process, losing a molecule of water in each step:
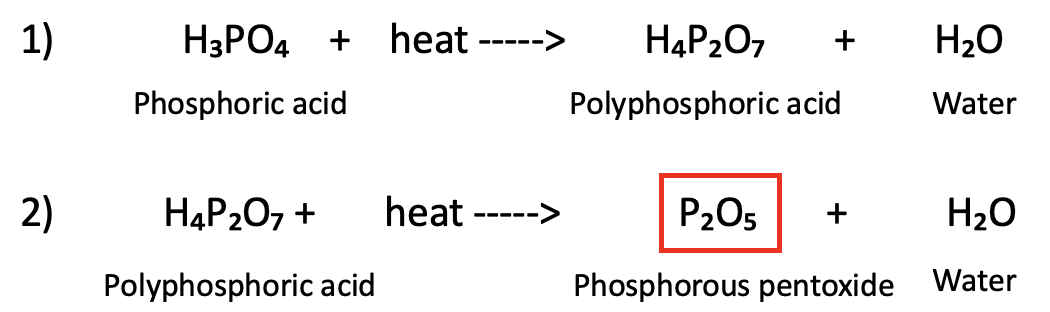
The phosphorous pentoxide (red box) is the workhorse; it has a very strong affinity for water, so much so that it can be used as a desiccant to protect moisture-sensitive chemicals from any water in the air. Here's a demonstration.
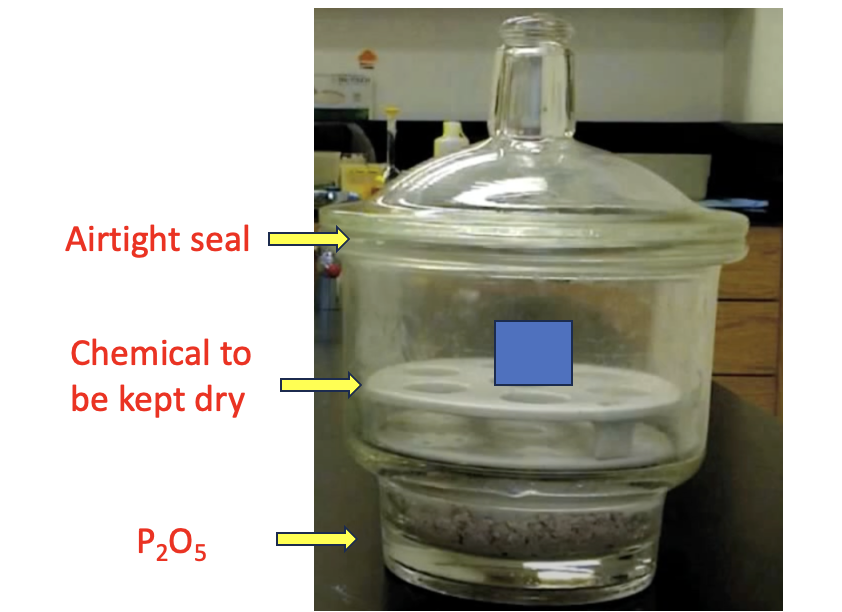
An example of a P2O5 desiccator. The desiccant is placed at the bottom, and the chemical that needs to be kept dry (blue box) sits on a tray above it. The top forms an airtight seal with petroleum jelly. And water that happens to get in there is immediately scavenged by the P2O5. Image: YouTube
Phosphorous pentoxide is so "thirsty" that sucks the water from the sugars that make up cellulose, the primary component of wood, leaving a char, which is resistant to burning.
To illustrate the char formation, check out this very entertaining YouTube video. It's simply an accelerated char process. Much stronger sulfuric acid (it will eat your face)is used instead of phosphoric acid, and sucrose (a simple two-sugar carbohydrate) is used in place of replaced cellulose, (3) the main component of wood. Think of this as "char on steroids."
Phosphoric acid recycles itself
What happens to the phosphoric acid once it has done its job? The essence of this fire retardant lies in a few well-designed and ingenious chemical reactions.
As phosphorus pentoxide reacts with cellulose, it forms a char layer that resists combustion. In the process, phosphorus pentoxide is converted back into phosphoric acid. When exposed to more heat, the phosphoric acid decomposes again (losing water) into phosphorus pentoxide, allowing the cycle to continue. This regenerative property ensures that the fire retardant remains effective as long as heat persists.
Bottom line
Although I have omitted some of the other components, e.g. clay, that give Phos-Chek95 more desirable properties (like thickness) the essence of the red liquid being dropped from planes is no more than a few simple, but clever, chemical reactions. Now you know why the red stuff is used. Let's hope that less and less of it will be required.
NOTES:
(1) The potential environmental concern of ammonium phosphate is that it is a fertilizer, which can promote the growth of algae and unwanted plants in waterways. While this issue has been controversial for some time now is not the time to discuss this.
(2) My colleague Chuck Dinerstein has written about the fires from a different angle.
(3) Cellulose is a huge polymer consisting of thousands of repeating glucose units.




